
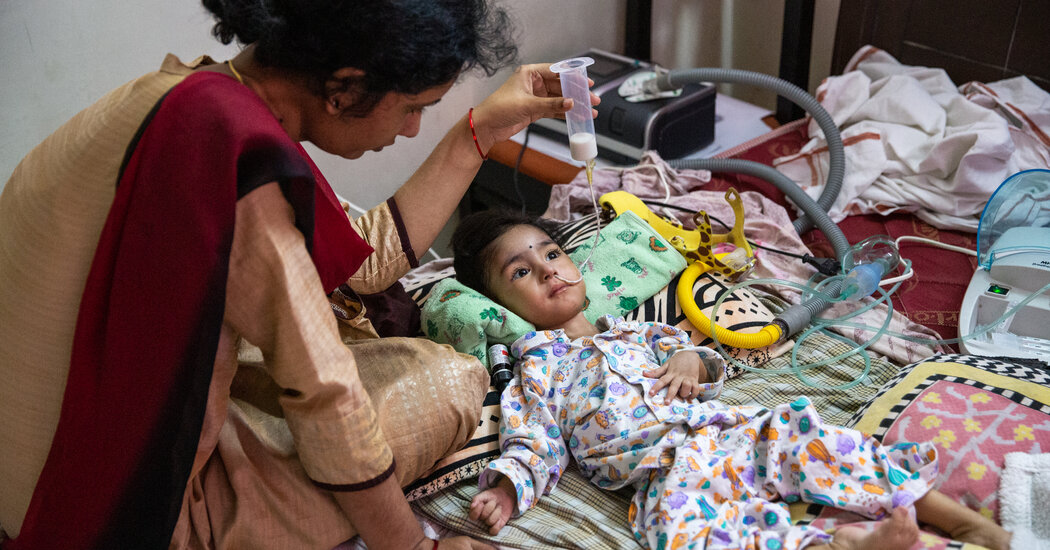
ELURU, India — When her baby started struggling to breathe, Stella Praveen had a terrible feeling that something was gravely wrong with her 14-month-old daughter, Ellen.
She ran barefoot to a nearby clinic, but the doctors there said the child needed to see a specialist right away. Without an ambulance, she jumped on the back of a motorcycle and rode 35 miles to a children’s hospital in another town, where Ellen remained in intensive care for 12 days.
Two weeks later, Ms. Praveen learned that her daughter, who had never been able to lift her neck nor roll over, was suffering from spinal muscular atrophy, a rare condition often fatal by age 2.
“We had not even heard of this disease,” Ms. Praveen said as tears rolled down her face. “She was misdiagnosed many times.”
The Praveen family was momentarily heartened when it learned that a promising gene therapy treatment was available, but was quickly crestfallen when it heard the cost: $2.1 million.
In India, and in many of the world’s poorer countries, the pharmaceutical industry’s latest advances for rare diseases are often agonizingly out of reach, impossible for almost all but the wealthiest families to afford and not covered by health insurance.
In desperation — and encouraged by the occasional success story — families are turning to social media to raise funds.
Every morning, Ellen’s father, Rayapudi Praveen, sends hundreds of emails on five crowdfunding websites like ImpactGuru and GoFundMe, asking people to contribute money to save his daughter’s life.
“Dear Sir, my daughter Ellen is suffering,” every email begins. “Can you help us?”
With only four months left before Ellen turns 2, time is running out — and the family is still far from its goal.
Spinal muscular atrophy is an inherited neuromuscular disease that kills more infants worldwide than any other genetic disorder. In India, one study put its prevalence at one in every 7,744 live births, or about 3,200 Indian babies each year.
Symptoms for all four types of the condition emerge at different stages. Infants like Ellen with Type 1, the most severe, show symptoms within their first six months of life: struggling to move their limbs, to swallow, to suck and eventually to breathe. They usually do not live past 2.
Across India, pediatric neurologists said, rising awareness among parents about the disease is leading to more patients being identified.
In recent years, India has established a reputation as a low-cost manufacturing hub for multinational pharmaceuticals, and the drugs made here are often substantially cheaper than imported ones, thanks in part to government price caps.
But the therapies for many rare diseases are still typically imported, forcing patients and parents to confront an excruciating truth: India’s status as a rising pharmaceutical superpower is of no help to them.
In 2019, the U.S. Food and Drug Administration approved the gene therapy Zolgensma, which alters the underlying genetic cause of spinal muscular atrophy and may permanently stop the disease’s progression.
At $2.1 million, the Zolgensma therapy from the pharmaceutical firm Novartis is believed to be the highest price ever set for a one-time treatment.
Spinraza, another drug, costs $750,000 in the first year and $375,000 a year after that, and needs to be taken for a lifetime.
April 6, 2022, 6:08 p.m. ET
Neither Zolgensma nor Spinraza, made by Biogen, is manufactured in India or approved for use here, so parents import them with the help of their doctors, a process that involves special government approvals.
The only drug approved for the condition in India is Evrysdi, manufactured by Roche. It’s the cheapest among the three treatments, but it still costs $53,000 to $80,000 a year, and that’s a discounted price for India, negotiated with Roche by the government.
None of these drugs are covered by insurance in India, so families face a wrenching choice: Raise the necessary money or see their children waste away.
So far, the Praveens have raised just over $100,000 for Ellen’s treatment, but they’re not giving up hope, and their optimism isn’t entirely unfounded.
Since May 2019, when Zolgensma was introduced, the parents of at least 10 children have succeeded in raising the $2.1 million through crowdfunding.
Last year, Yogesh Gupta started a crowdfunding campaign and sent emails to anyone he knew asking for help for his son, Ayaansh, who has Type 1. Soon a team of 125 friends, colleagues and relatives began sending messages on social media platforms to politicians and Bollywood stars. Moved by the plight of the child, the officials and celebrities not only donated money themselves but also helped spread the word.
After three and a half months, Mr. Gupta said he raised $2.1 million.
“There is a lot of improvement,” Mr. Gupta said of his son after he received the Zolgensma treatment. “He can slightly lift his legs and neck control is far better.”
Raman Nagumantri is more than halfway there, having raised $1.6 million for his 19-month-old daughter, Khyati.
“We don’t remember a day when we have slept for the whole night since she was diagnosed,” Mr. Nagumantri said. “But we are close, and I can do anything, anything, to get those required funds in these four months.”
For almost all the world’s children with Type 1, their best chance of survival may lie with the Global Managed Access Program, or gMAP, which provides Zolgensma for free to a select number of eligible patients under the age of 2 in countries where the gene therapy has not received regulatory approval or is not covered by insurance.
Representatives from Novartis said more than 250 children from around the world had received the therapy free through gMAP.
Novartis declined to share the total number of patients from India, but Dr. Ann Mathew, a leading pediatric neurologist, who has over 400 spinal muscular atrophy patients, said 40 children had received the treatment in the past year across India, the majority through gMAP. Nineteen of her patients have taken Zolgensma in the past 13 months, 16 free and three paid.
Biogen said 200 patients in India had received Spinraza for free.
Patient advocacy groups are pushing for government intervention to negotiate better prices with pharmaceutical companies.
“When the government intervenes, the prices will automatically go down,” said Alpana Sharma, co-founder of Cure SMA, a parent-led advocacy group. “This is what happened with cancer and other rare diseases like hemophilia.”
While the parents of children with Type 1 face a harrowingly short time period for a cure, the treatments for Type 2, which has debilitating effects but is not typically fatal before adulthood, are also far beyond the means of most caregivers.
In the coastal state of Goa, Ruby Borges and her husband, Benedict Borges, were devastated when their 5-year-old son, Dylan, was diagnosed three years ago with S.M.A.-Type 2. In most cases, Type 2 symptoms arrive between six and 18 months, and children suffering from it cannot walk.
At the time of Dylan’s diagnosis, Spinraza was the only treatment available.
After he didn’t get into the compassionate access program, his parents turned to crowdfunding. Months of appeals made through church groups in their community helped collect $57,000. At that rate, it would take years to source the money to pay for Spinraza, and in the meantime, Dylan was growing weaker as his muscles atrophied.
Doctors advised Dylan’s parents to start him on Evrysdi. They managed to buy enough supply of that drug to last through the end of the year. Dylan’s mother feels that the medication and intense physiotherapy are helping, and she said she had seen a 20 percent improvement in his condition. But she worries about how long she can keep relying on the generosity of strangers to keep her son alive.
“People laugh when they hear the price of the medicine,” Ms. Borges said. “They wonder if I’m going to spend it on a car or a big house.”
The parents of Ellen need even more money and have even less time.
On a recent afternoon, her father walked down a dirt road toward a nearby highway, where he hitched a ride to the city of Vijayawada. Hours later, he arrived at the large house of a businessman-philanthropist he hoped could help.
But it was not to be.
“Accept your fate and move on,” the businessman told him.
Mr. Praveen looked through a window onto the businessman’s sprawling lawn and vowed not to give up.
“I will fight to her last breath,” he said.

More Stories
Ronny J and Branden Condy were recently spotted together in front of LIV club in Miami Beach, FL
Abu Dhabi Sustainability Week to host first Green Hydrogen Summit
IDEX, NAVDEX to showcase fast-changing defence sector